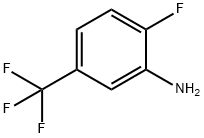
Methyl butyrate(CAS#623-42-7)
| Likhoutu tsa Kotsi | R20 – E kotsi ka ho hema R36/37/38 – Ho tena mahlo, tsamaiso ya ho hema le letlalo. R11 – E tuka Haholo |
| Tlhaloso ea Tšireletseho | S16 - Ipoloke hole le mehloli ea mollo. S26 - Ha u kopana le mahlo, hlatsoa hang-hang ka metsi a mangata 'me u batle keletso ea bongaka. S36 - Apara liaparo tse loketseng tsa tšireletso. S33 - Nka mehato ea boits'ireletso khahlanong le ho tsoa ho sa fetoheng. S29 - Se ke oa tšela ka har'a li-drain. S9 - Boloka setshelo sebakeng se nang le moea o motle. |
| Li-ID tsa UN | UN 1237 3/PG 2 |
| WGK Jeremane | 2 |
| RTECS | ET5500000 |
| LIKHOUTU FLUKA BRAND F | 13 |
| TSCA | Ee |
| Khoutu ea HS | 29156000 |
| Sehlopha sa Likotsi | 3 |
| Sehlopha sa ho paka | II |
Selelekela
Methyl butyrate. Se latelang ke selelekela ho tse ling tsa thepa, tšebeliso, mekhoa ea ho lokisa le tlhaiso-leseling ea polokeho ea methyl butyrate:
Boleng:
- Methyl butyrate ke mokelikeli o tukang o sa qhibiliheng ka metsing.
- E na le monyetla o motle, o qhibilihang ka har'a lino tse tahang, li-ethers le lihlapolli tse ling tsa tlhaho.
Sebelisa:
- Methyl butyrate e atisa ho sebelisoa e le solvent, plasticizer le diluent ka lijalo.
- E ka boela ea sebelisoa e le karolo e bohareng ea organic synthesis bakeng sa ho lokisa metsoako e meng.
Mokhoa:
- Methyl butyrate e ka lokisoa ka ho arabela butyric acid ka methanol tlas'a maemo a acidic. Reaction equation ke e latelang:
CH3COOH + CH3OH → CH3COOCH2CH2CH3 + H2O
- Karabelo hangata e etsoa ka ho futhumatsa ka sesebelisoa (mohlala, sulfuric acid kapa ammonium sulfate).
Lintlha tsa Tšireletseho:
- Methyl butyrate ke mokelikeli o tukang o ka tukang ha o pepesehile malakabe a bulehileng, mocheso o phahameng kapa li-organic oxidants.
- Ho kopana le letlalo le mahlo ho ka baka ho teneha le ho chesa, ho lokela ho nkoa mehato ea ho itšireletsa.
- Methyl butyrate e na le chefo e itseng, kahoo e lokela ho qojoa bakeng sa ho inhalation le ho kenngoa ka phoso, 'me e sebelisoe tlas'a maemo a nang le moea o motle.
- Tlhokomelo e lokela ho nkoa ho thibela ho kopana le li-oxidants, acid le alkalis ha u sebelisa kapa u boloka.