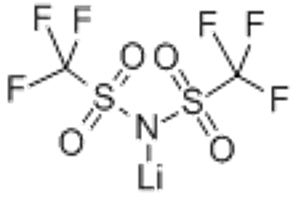Lithium bis(trifluoromethanesulphonyl)imide(CAS# 90076-65-6)
| Likhoutu tsa Kotsi | R24/25 - R34 – E baka ho cha R52/53 – E kotsi ho dibopuwa tsa metsing, e ka baka ditlamorao tse mpe tsa nako e telele tikolohong ya metsing. R48/22 – Kotsi e kotsi ya tshenyo e kgolo ya bophelo bo botle ka ho ba pepeneneng nako e telele ha motho a kwetswe. |
| Tlhaloso ea Tšireletseho | S22 - U se ke ua hema lerole. S26 - Ha u kopana le mahlo, hlatsoa hang-hang ka metsi a mangata 'me u batle keletso ea bongaka. S36/37/39 - Apara liaparo tse loketseng tsa tšireletso, liatlana le tšireletso ea mahlo / sefahleho. S45 – Ha ho ka etsahala kotsi kapa ha o ikutlwa o sa phele hantle, batla keletso ya bongaka hanghang (bontsha leibole neng kapa neng ha ho kgoneha.) S61 - Qoba ho lokolloa tikolohong. Sheba litaelo tse khethehileng / maqephe a tšireletso. |
| Li-ID tsa UN | UN 2923 8/PG 2 |
| WGK Jeremane | 2 |
| TSCA | Ee |
| Khoutu ea HS | 29309090 |
| Tsebiso ea Kotsi | E Kotsi/E senyang/E Mamela Mongobo |
| Sehlopha sa Likotsi | 8 |
| Sehlopha sa ho paka | II |
Selelekela
Lithium bis-trifluoromethane sulfonimide. Se latelang ke selelekela sa sebopeho sa eona, tšebeliso, mekhoa ea tlhahiso le tlhaiso-leseling ea polokeho:
Boleng:
Lithium bis-trifluoromethane sulfonimide ke kristale e se nang mebala kapa phofo e tšoeu ea kristale, e nang le botsitso bo phahameng ba mocheso le lik'hemik'hale. E qhibiliha ka li-solvents tse seng polar tse kang ether le chloroform mocheso oa kamore, empa ho thata ho qhibiliha ka metsing.
Sebelisa:
Lithium bis-trifluoromethane sulfonimide e sebelisoa haholo tšimong ea organic synthesis. E ka sebelisoa e le mohloli oa litsamaiso tse matla tsa asiti le organic synthesis, joalo ka mehloli ea fluoride ion le li-catalyst tsa alkali lits'ebetsong tse matla tsa alkaline. E ka boela ea sebelisoa e le tlatsetso ea electrolyte ka libeteri tsa lithium-ion.
Mokhoa:
Ho lokisoa ha lithium bis-trifluoromethane sulfonimide hangata ho fumanoa ka ho arabela trifluoromethane sulfonimide ka lithium hydroxide. Trifluoromethane sulfonimide e qhibiliha ka har'a motsoako oa polar, ebe lithium hydroxide e eketsoa ho hlahisa lithium bistrifluoromethane sulfonimide nakong ea karabelo, 'me sehlahisoa se fumanoa ka ho tsepamisa mohopolo le crystallization.
Lintlha tsa Tšireletseho:
Lithium bis-trifluoromethane sulfonimide hangata e bolokehile tlas'a maemo a tloaelehileng a tšebeliso, empa ho ntse ho e-na le lintho tse 'maloa tseo u lokelang ho li hopola:
- Lithium bistrifluoromethane sulfonimide e ka baka ho teneha ha mahlo le letlalo, 'me ho kopana ka ho toba ho lokela ho qojoa nakong ea ho tšoaroa.
- Mehato e nepahetseng ea moea e lokela ho nkoa ha ho tšoaroa, ho boloka, kapa ho lahla lithium bistrifluoromethane sulfonimide ho netefatsa polokeho.
- Ha e futhumetse kapa e pepesehetse mocheso o phahameng, lithium bistrifluoromethane sulfonimide e kotsing ea ho phatloha 'me e lokela ho qojoa hore e se ke ea kopana le malakabe a bulehileng kapa mocheso o phahameng.
- Ha o sebelisa lithium bis-trifluoromethane sulfonimide, latela mekhoa e nepahetseng ea ts'ebetso ea ts'ireletso 'me u apare lisebelisoa tse nepahetseng tsa ts'ireletso joalo ka liatlana, likhalase le liaparo tsa ts'ireletso.