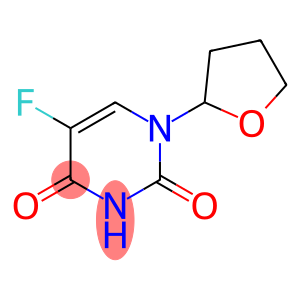
Ftorafur (CAS#17902-23-7)
| Matšoao a Kotsi | T - e chefo |
| Likhoutu tsa Kotsi | 23/24/25 - Chefo ka ho hema, ho kopana le letlalo le haeba e metsoa. |
| Tlhaloso ea Tšireletseho | S22 - U se ke ua hema lerole. S36/37/39 - Apara liaparo tse loketseng tsa tšireletso, liatlana le tšireletso ea mahlo / sefahleho. S45 – Ha ho ka etsahala kotsi kapa ha o ikutlwa o sa phele hantle, batla keletso ya bongaka hanghang (bontsha leibole neng kapa neng ha ho kgoneha.) |
| Li-ID tsa UN | UN 2811 6.1/PG 2 |
| WGK Jeremane | 3 |
| RTECS | YR0450000 |
| Khoutu ea HS | 29349990 |
| Sehlopha sa Likotsi | 6.1 |
| Sehlopha sa ho paka | II |
| Chefo | LD50 ka litoeba (mg / kg): 900 ka molomo (matsatsi a 3) (Yasumoto); 750 ip (FR 1574684), hape e tlalehiloe e le 1150 ip (Smart) |
Selelekela
Trifluoromethylation ke organic chemical reaction eo ho eona lihlopha tsa trifluoromethyl li ka kenngoa ka har'a limolek'hule tse sebelisang li-reagents tsa tegafluor tse kang TMSCF3.
Lintho tse molemo tsa tegafluor:
- Tegafluor ke karabelo ea bohlokoa ea phetoho ea sehlopha, e ka hlahisang lihlopha tsa trifluoromethyl ka sekhahla se itseng sa elektronike ho fetola thepa ea 'mele le ea lik'hemik'hale ea limolek'hule.
- Lihlopha tsa Trifluoromethyl li na le khoheli e matla ea elektronike, e ka eketsang electrophilicity ea molek'hule le ho qhibiliha ha solvent.
- Lihlahisoa tsa tegafluor reaction hangata li tsitsitse lik'hemik'hale 'me li sebetsa ka baeloji.
Tšebeliso ea tegafluor:
- Sebakeng sa mahlale a thepa, tegafluor e ka fetola thepa ea thepa, ea eketsa botsitso le ho hanyetsa boemo ba leholimo.
Mokhoa oa ho lokisa tegafluor:
- Li-reagents tsa tegafluor tse sebelisoang ka tloaelo li kenyelletsa: TMSCF3, Ruppert-Prakash reagent, joalo-joalo.
- Karabelo ea tegafluor hangata e etsoa sebakeng se sa sebetseng, ho sebelisoa motsoako oa inert (mohlala, methylene chloride, chloroform) joalo ka mokhoa oa ho arabela.
- Maemo a karabelo ka kakaretso a hloka mocheso o phahameng oa karabelo le nako e telele ea karabelo, 'me hangata a hloka ho eketsoa ha "catalyst" (mohlala, copper catalyst).
Lintlha tsa polokeho ho tegafur:
- Li-reagents tsa Tegafluor li chefo ebile lia senya, 'me ho hlokahala hore ho nkoe mehato e nepahetseng ha e sebelisoa.
- Likhase (mohlala, hydrogen fluoride) tse hlahisoang nakong ea karabelo le tsona li kotsi 'me li hloka ho sebetsoa ka tlas'a maemo a nang le moea o motle.
- Tlhokomelo e lokela ho nkoa ho qoba ho kopana le metsi kapa mongobo nakong ea ts'ebetso ho qoba liphetoho tse ke keng tsa fetoha tsa lik'hemik'hale.
- Li-reactants le lihlahisoa tlas'a maemo a tegafluor reaction li hloka kalafo e nepahetseng le ho lahla litšila.